Electrodialysis is an electro-membrane process in which a direct electric field influences the movement of dissociated components of salts in an aqueous solution so that cations moving towards the cathode are transmitted through cation-exchange membranes and held by anion-exchange membranes, while anions attracted towards the anode are transmitted through anion-exchange membranes and held on cation-exchange membranes.
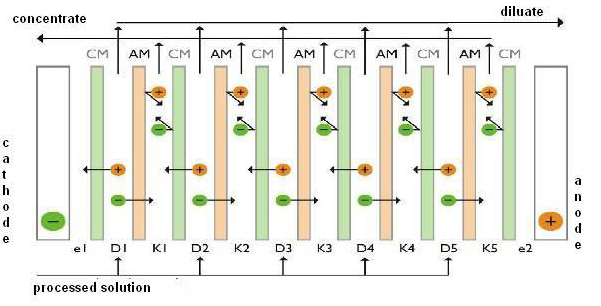
By a proper combination of cation- and anion-exchange membranes, ions in the inflow solution are separated, generating the desalinated flow called the diluate and the concentrated flow called the concentrate. This separation is achieved due to the influence of the electric field and on the basis of the different permselectivity of ion-exchange membranes for individual components in the solution. By both electrodes, so-called electrode solution circulates (usually a solution of indifferent salt or inflow treated solution), washing their surface regularly and not participating in the electrodialysis separation.
The goal of electrodialysis can be acquiring products of one type of solution only (diluate or concentrate); however, in some cases there are quality requirements concerning both products (especially in waste water treatment), e.g. when the diluate must meet criteria for being released into the watercourse and the concentrate must have the best possible parameters for further processing.



