Diffusion dialysis (DD) is an ion-exchange membrane separation process which can be applied for separation and recovery of free acid from waste solutions containing free acid contaminated by their salts.
Separation principle
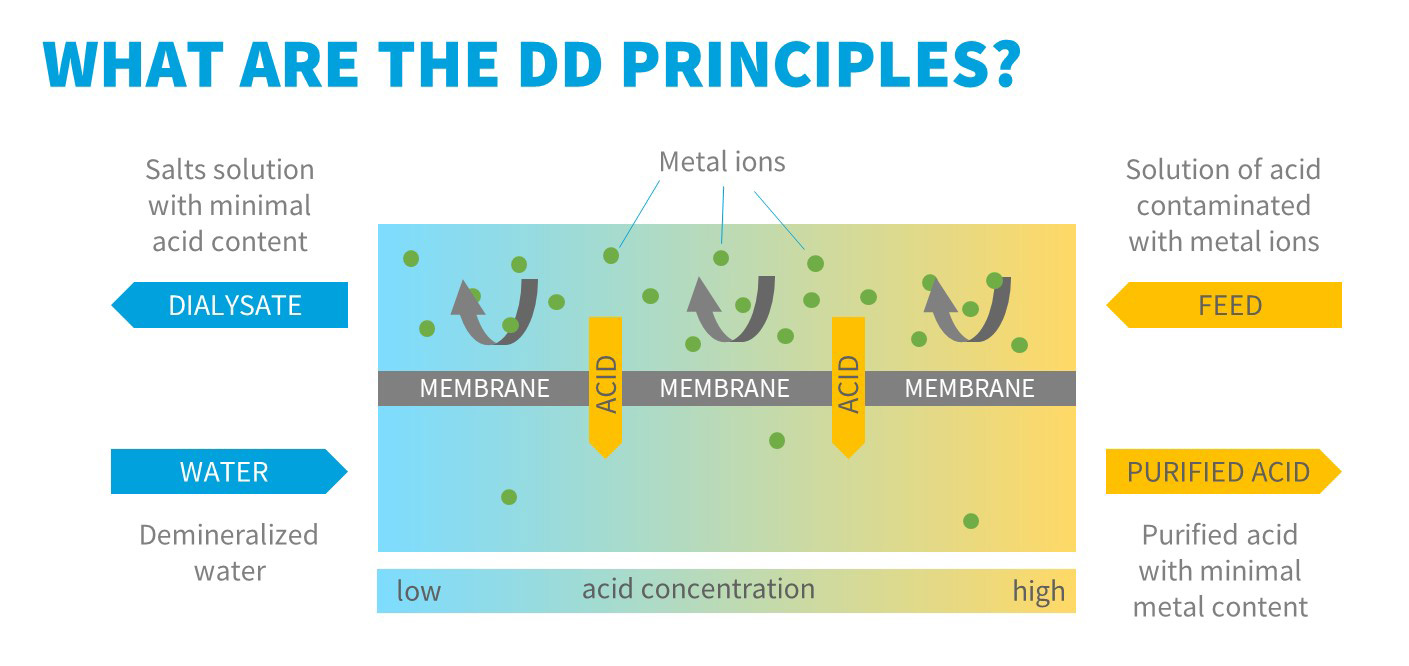
In the acid dialysis process the anion exchange membranes are used. The membranes wear fixed positive charge and therefore they attract negatively charged ions while the positively charged ions are repulsed.
Two streams enter the module. The first solution contains free acid contaminated with its salts, the second inlet stream contains demineralized water. The concentration gradient between the two solutions is the driving force of the process. The anions migrate easily through the membrane. Due to very high mobility the hydrogen ion can pass the membrane together with the anions to maintain the electroneutrality of the solution. The metal cations are however retained in the original solution due to their positive charges and repulsion by the membrane.
Thus, the acid passes preferentially through the membrane. Due to the non-ideality of the membranes, a part of metal cations also pass the membrane, but in significantly lower extent. The purified acid leaves the module as the so called diffusate, a waste stream containing majority of the metal ions as the so called dialysate.
After purification, the diffusate contains about 90 % of the total amount of free acid originally present in the feed solution, the remaining free acid remains in the dialysate. The concentration of metal cations in the recycled acid drops ca 20 times in one pass technology.
Main advantages of the technology
The technology is robust and economically and environmentally beneficial:
- Approximately 90% of free acid can be recycled from waste streams.
- This leads to reduction of the cost for purchasing of the fresh acid. The consumption of the neutralizing agent for neutralizing the free acid in the waste also decreases by approx. 90 %.
- If precipitation occurs during the waste neutralization (typically when neutralizing waste sulfuric acid with lime hydrate), up to 90 % less solid waste (often defined as hazardous) is generated. This leads both to a reduction in the negative impact of production on the environment and to significant financial savings for waste disposal. This benefit can have a significant effect on the overall financial balance sheet.
- Diffusion dialysis can also be used to reduce the salt content of wastewater if the salts are soluble and discharged to the sewerage after neutralization. This leads to a reduction in the burden on the environment and can lead to economic benefits.
- The process can be designed as continuous or batch according to customer needs.
- In the case of diffusion dialysis, it is an energy-efficient process.
- Technology is easily scalable thanks to the modularity of the design solution.
Typical applications
A typical application of diffusion dialysis is the regeneration of sulfuric acid from an anodizing bath (anodic oxidation of aluminum). However, it can also be used in other processes, where the concentration of waste acids is in a significant amount (separation of acid from heavy / rare metals from leaching, pickling, surface treatment and others).



